- Home
- About Us
-
Products
.png)
-
Application
.png)
-
Blog
.png)
- Contact us

How Magnesium Hydroxide Affects Polymer Rheology and Processing
The global demand for halogen-free flame retardant (HFFR) materials has accelerated dramatically as industries worldwide respond to stricter environmental regulations and heightened safety requirements. Wire and cable manufacturers, automotive component producers, and construction material suppliers are increasingly transitioning away from halogen-based additives toward cleaner alternatives that deliver comparable flame suppression without the toxic byproducts associated with brominated or chlorinated compounds. Within this landscape, magnesium hydroxide (MDH) has emerged as one of the most widely adopted inorganic flame retardants, prized for its endothermic decomposition mechanism, effective smoke suppression capabilities, and favorable environmental profile. However, incorporating MDH into polymer matrices presents significant technical challenges that polymer engineers and compounding specialists must carefully navigate.
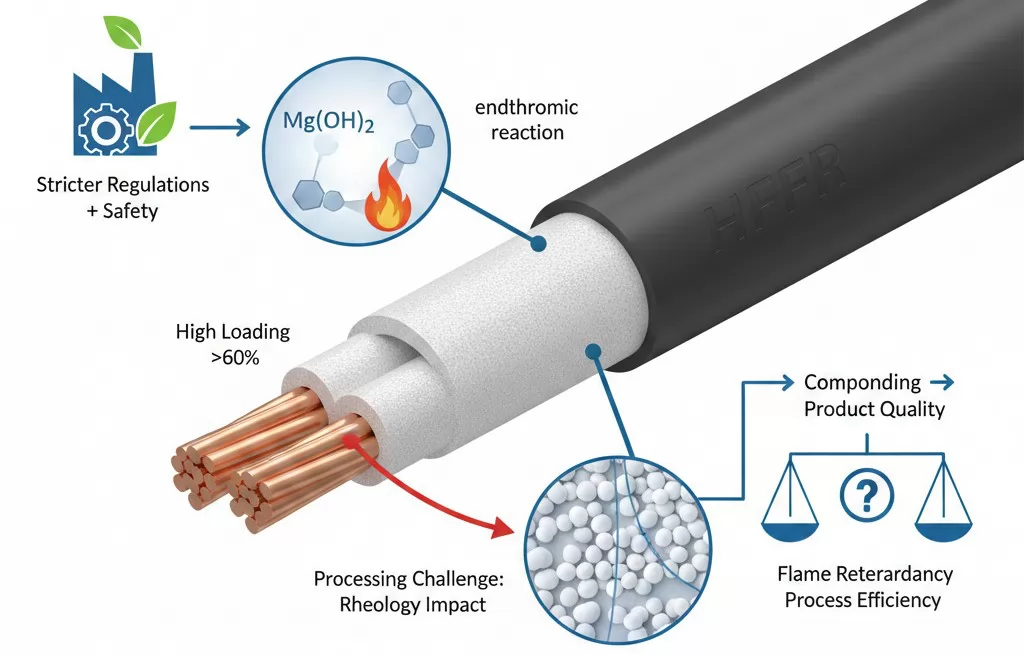
The fundamental challenge lies in the paradox at the heart of MDH flame retardant formulation: achieving effective fire protection typically requires high loading levels often exceeding 60 percent by weight, yet these same high loadings dramatically alter the flow behavior and processing characteristics of the base polymer. Understanding how magnesium hydroxide affects polymer rheology is essential for manufacturers seeking to balance flame retardancy performance with production efficiency, product quality, and cost-effectiveness. This article examines the technical mechanisms behind MDH's rheological effects, explores practical processing implications, and presents actionable solutions that enable manufacturers to optimize their compounding and forming operations while maintaining target material properties.
Understanding Magnesium Hydroxide in Polymer Matrices
Magnesium hydroxide, chemically represented as Mg(OH)₂ and commonly derived from natural brucite ore or produced through synthetic processes, functions as a halogen-free flame retardant through a dual-action mechanism that combines physical and chemical fire suppression. When exposed to temperatures exceeding approximately 300-340°C, MDH undergoes endothermic decomposition that absorbs heat from the surrounding environment while releasing water vapor. This decomposition process:
- Absorbs approximately 1,400 J/g of heat energy
- Releases water vapor that dilutes flammable gases
- Forms a protective magnesium oxide residue layer
- Creates a thermal barrier between polymer and flame
The effectiveness of magnesium hydroxide as a flame retardant depends critically on the loading level achieved within the polymer matrix. Unlike some organic flame retardant additives that achieve their protective effects at concentrations of just a few percent, MDH requires substantially higher loading levels to reach comparable flame suppression performance. For many applications, formulators typically incorporate MDH at specific loading ranges:
| Target Rating | Typical MDH Loading | Applications |
|---|---|---|
| UL94 V-1 | 45-55% | General electrical enclosures |
| UL94 V-0 | 55-65% | Wire insulation, connectors |
| LOI > 30% | 60-75% | Building materials, transportation |
| Extreme fire protection | 75-80% | Tunnel cables, specialty applications |
The implications of these high loading levels for polymer processing are significant. When 60 percent or more of the compound volume consists of solid mineral particles, the resulting material behaves fundamentally differently from the unfilled polymer. The rheological properties shift dramatically, affecting everything from melt viscosity and shear response to thermal stability and mechanical characteristics.
The Rheological Impact: Viscosity and Flow Behavior
Melt Viscosity Enhancement
The addition of magnesium hydroxide to polymer matrices produces substantial increases in melt viscosity that directly impact processing behavior across all major forming operations. When unfilled polymer flows, the long polymer chains slide past one another relatively freely, allowing the material to deform and fill molds under applied stress. Introducing solid MDH particles creates physical barriers that impede chain mobility, requiring greater force to achieve equivalent flow rates. At the high loading levels necessary for effective flame retardancy, this viscosity enhancement can increase melt resistance dramatically.
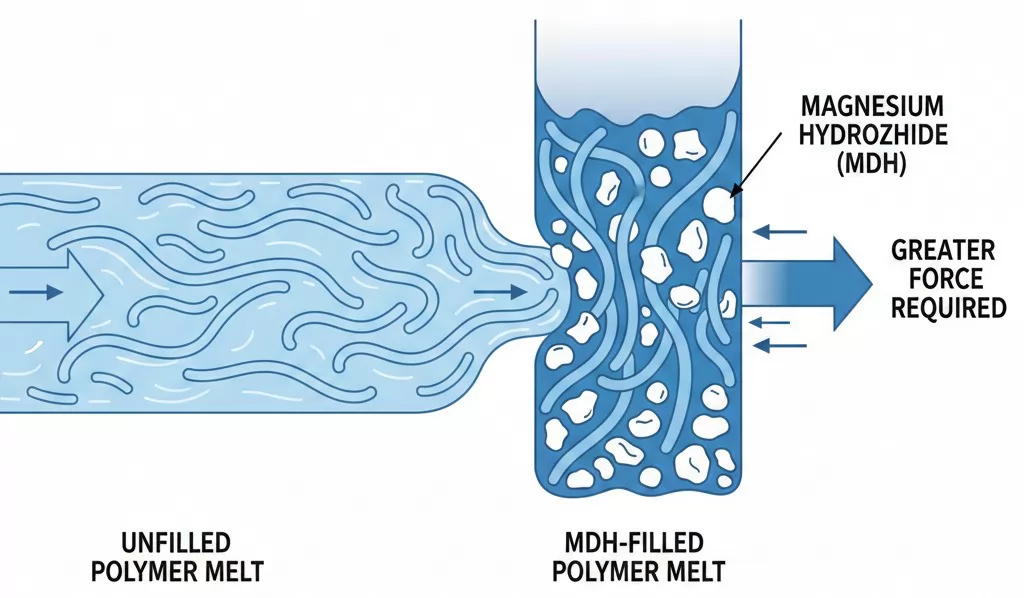
The magnitude of viscosity increase correlates strongly with the volume fraction of MDH particles rather than simply their weight percentage. Key factors influencing viscosity include:
- Particle concentration: As volume fraction exceeds 25-30%, particle-particle interactions dominate flow behavior
- Particle shape: Irregular MDH particles create more resistance than spherical fillers
- Particle size: Finer particles increase surface area and interparticle contacts
- Surface treatment: Coated particles reduce aggregation and lower effective volume
Research examining the rheological behavior of MDH-filled compounds has demonstrated that the relationship between filler loading and viscosity is non-linear. A typical progression might look like:
| MDH Loading (wt%) | Relative Viscosity Increase | Processing Difficulty |
|---|---|---|
| 30% | 1.5-2x | Minor adjustments needed |
| 50% | 3-5x | Moderate modifications required |
| 60% | 8-15x | Significant equipment changes |
| 70% | 15-25x+ | Specialized processing essential |
Shear Thinning Behavior and Processing Response
Despite the elevated baseline viscosity, MDH-filled polymer compounds typically exhibit pronounced shear thinning behavior that provides important processing advantages under the high-shear conditions encountered in extrusion and injection molding operations. Shear thinning refers to the phenomenon whereby apparent viscosity decreases as shear rate increases.
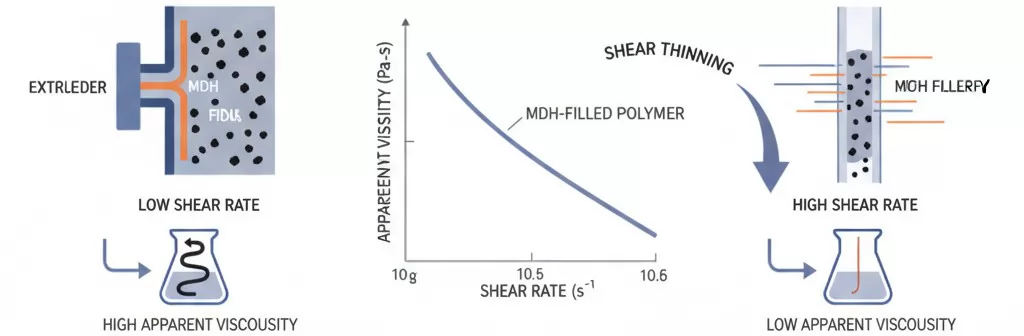
The shear thinning response varies significantly based on particle characteristics:
| MDH Type | Shear Sensitivity | Processing Characteristics |
|---|---|---|
| Untreated, coarse | Poor | High viscosity across all shear rates, difficult processing |
| Untreated, fine | Moderate | High low-shear viscosity, some improvement at high shear |
| Surface-treated | Good | Significant viscosity reduction at processing shear rates |
| Nano-sized, treated | Excellent | Wide processing window, complex geometry capability |
Understanding the shear rate profiles encountered in specific processing operations is essential for optimizing MDH compound formulation:
- Calendering: 10-100 s⁻¹
- Extrusion (low shear): 100-500 s⁻¹
- Extrusion (high shear): 500-1,000 s⁻¹
- Injection molding: 1,000-10,000+ s⁻¹
Yield Stress and Solid-Like Behavior
One of the most consequential rheological phenomena associated with highly filled MDH compounds is the emergence of yield stress behavior, wherein the material exhibits solid-like characteristics at low stress levels and only begins to flow once applied stress exceeds a critical threshold. This yield stress arises from the formation of percolating particle networks.
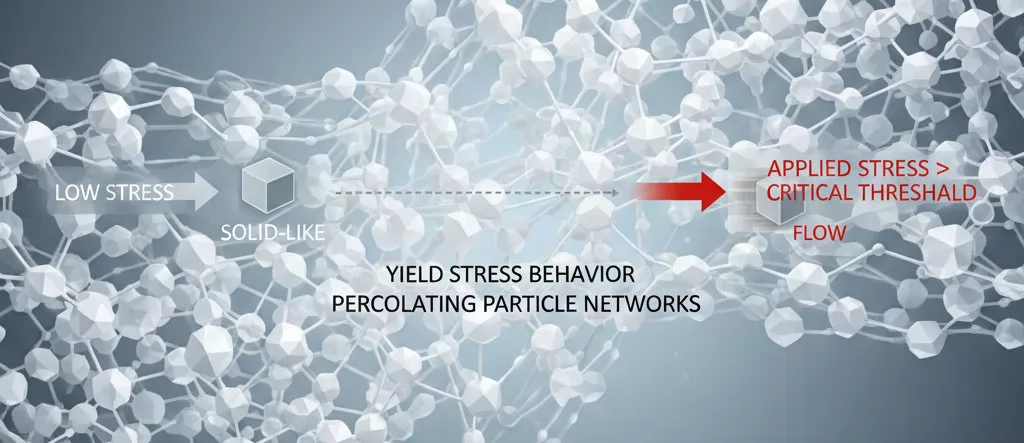
Practical implications of yield stress include:
- Feeding difficulties: Material may resist flow into extruder feed throat
- Dead zones: Accumulation in equipment corners with low stress
- Process instability: Erratic pressure readings and output variations
- Start-up challenges: Extended time to achieve steady-state flow
Processing Implications for Manufacturers
Extrusion Challenges and Solutions
The extrusion of MDH-filled compounds presents a distinct set of processing challenges that require careful attention to equipment configuration, temperature management, and screw design. Compounds with high filler loadings generate significantly higher back pressure within the die as the viscous melt forces its way through the constricted flow channel.
Key extrusion processing parameters to monitor and optimize:
| Parameter | Typical Impact | Recommended Action |
|---|---|---|
| Screw torque | 50-100% higher than unfilled | Use high-torque drive systems |
| Die pressure | 2-4x increase | Reinforce die components |
| Motor load | 30-60% increase | Size motor appropriately |
| Throughput rate | 20-40% reduction | Adjust line speed expectations |
| Screw wear | Accelerated | Use hardened barrel/screw surfaces |
Surface finish quality represents another critical concern during MDH compound extrusion. Surface defects commonly encountered include:
- Sharkskin roughness: Melt fracture at die exit due to high elasticity
- Particle protrusion: Inadequate dispersion causing visible particles
- Flow lines: Inconsistent flow patterns creating visible marks
- Die swell variation: Unstable extrudate expansion
Mitigation strategies include:
- Optimizing die land length and exit angles
- Applying appropriate surface treatments to MDH particles
- Using mixing sections for better dispersion
- Controlling temperature profiles precisely
Injection Molding Considerations
Injection molding of MDH-filled compounds requires particular attention to mold filling behavior and the relationship between flow length and part geometry. The elevated viscosity and pronounced shear sensitivity of these compounds affect how readily the melt advances through mold cavities.
Critical injection molding adjustments for MDH compounds:
| Parameter | Standard Setting | MDH Compound Adjustment |
|---|---|---|
| Injection pressure | Baseline | +30-50% higher |
| Pack pressure | Baseline | +20-40% higher |
| Hold time | Baseline | +50-100% longer |
| Cooling time | Baseline | +10-20% longer |
| Mold temperature | Baseline | +10-20°C higher |
Design considerations for MDH-filled injection molded parts:
- Increase gate sizes to reduce flow resistance
- Reduce wall thickness to improve flow length
- Optimize gate location for balanced filling
- Consider multiple gates for large parts
- Allow for increased shrinkage in thick sections
Thermal Stability and Processing Window
The thermal decomposition profile of magnesium hydroxide fundamentally shapes the available processing window for MDH-filled compounds. MDH provides a substantial thermal safety margin for most polymer processing operations.
| Flame Retardant | Decomposition Range | Max Processing Temp | Compatible Polymers |
|---|---|---|---|
| ATH (Aluminum Trihydrate) | 180-280°C | 200-220°C | PE, PP, PVC |
| MDH (Magnesium Hydroxide) | 300-340°C | 280-320°C | PE, PP, PA, PBT |
| Zinc Borate | 250-300°C | 260-280°C | Engineering plastics |
Advantages of MDH's higher decomposition temperature:
- Processing temperatures 50-100°C higher than ATH equivalents
- Compatible with engineering polymers (polyamides, PBT)
- Reduced risk of premature flame retardant degradation
- Wider processing window for temperature variations
Mitigating Processing Issues: Technical Solutions
Surface Treatment Technologies
Surface modification of magnesium hydroxide represents the most effective strategy for improving the processing characteristics of MDH-filled compounds. The native surface of MDH particles is hydrophilic, creating strong tendencies toward aggregation in non-polar polymer matrices.
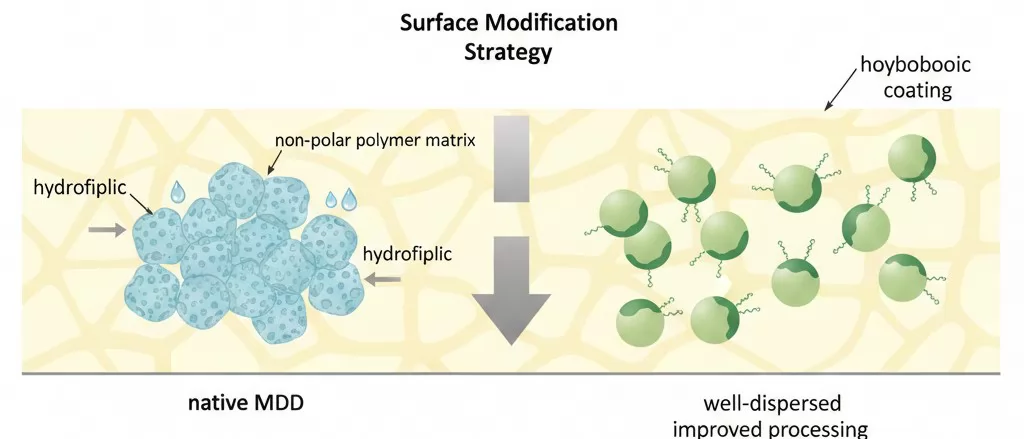
| Treatment Type | Examples | Benefits | Limitations |
|---|---|---|---|
| Silane coupling | A-174, amino-silanes, epoxy-silanes | Chemical bonding, excellent dispersion, property enhancement | Higher cost, requires precise application |
| Fatty acids | Stearic acid, oleic acid, magnesium stearate | Low cost, easy application, effective viscosity reduction | No chemical bonding, limited property improvement |
| Polymer coatings | PE wax, EVA copolymers | Improved compatibility, reduced abrasion | May affect flame retardancy |
Benefits of surface treatment are well-documented and substantial:
- Viscosity reduction: 30-50% lower than untreated MDH at same loading
- Improved dispersion: More uniform particle distribution
- Enhanced mechanical properties: Better stress transfer at interface
- Stable processing: Consistent behavior over time
Particle Size Distribution Optimization
The particle size distribution of magnesium hydroxide significantly influences the rheological behavior of filled compounds. Optimizing particle packing can reduce the effective volume fraction and decrease compound viscosity.
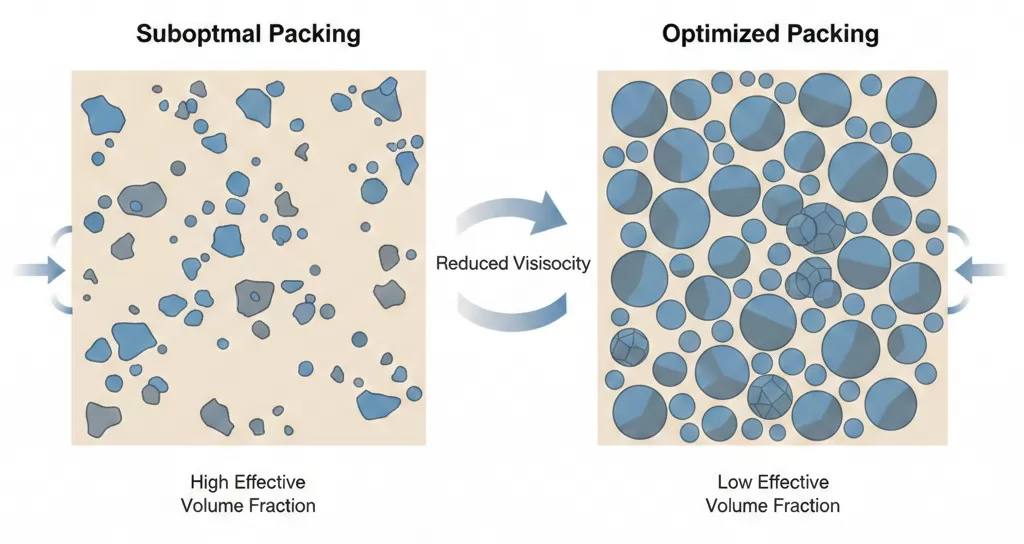
Particle size distribution strategies:
| Strategy | Description | Viscosity Effect |
|---|---|---|
| Unimodal, narrow | Single particle size distribution | Higher viscosity, poor packing |
| Bimodal | Blend two different sizes | 15-25% viscosity reduction |
| Trimodal | Blend three different sizes | 25-35% viscosity reduction |
| Continuous distribution | Wide, optimized distribution | Maximum reduction possible |
Practical considerations for particle size optimization:
- Finer particles improve flame retardant effectiveness but increase viscosity
- Surface treatment becomes more important at smaller particle sizes
- Coarser particles reduce processing difficulty but may compromise properties
- Blending grades can balance multiple requirements
Processing Aid Additives
Specialized processing aid additives offer additional options for managing the rheology of MDH-filled compounds:
| Additive Type | Function | Application |
|---|---|---|
| Fluoropolymers | Reduce melt fracture, improve surface finish | Wire extrusion, film production |
| Internal lubricants | Reduce polymer chain friction | General compounding, injection molding |
| External lubricants | Reduce equipment adhesion | High output extrusion |
| Rheology modifiers | Adjust flow behavior | Specialty applications |
Practical Applications and Industry Applications
Wire and Cable Applications
The wire and cable industry represents one of the largest and most demanding application areas for magnesium hydroxide flame retardant compounds. Low smoke zero halogen (LSZH) cable compounds routinely incorporate 55-65 percent MDH to achieve fire safety performance demanded by building codes and transportation standards.
LSZH cable compound processing requirements:
- Extruder configuration: High compression ratio screws, mixing sections
- Temperature profile: 150-190°C zones, reduced feed zone
- Line speed: 100-300 m/min typical
- Cooling: Multi-stage water tanks with controlled graduation
Quality considerations for cable applications:
- Surface finish: No sharkskin, particles, or flow lines
- Dimensional control: ±0.05mm tolerance typical
- Density consistency: Uniform across production runs
- Flame test performance: Consistent UL94/FT4 ratings
Automotive Components
Automotive applications increasingly incorporate MDH flame retardant compounds to meet evolving safety requirements while avoiding halogenated additives facing regulatory scrutiny.
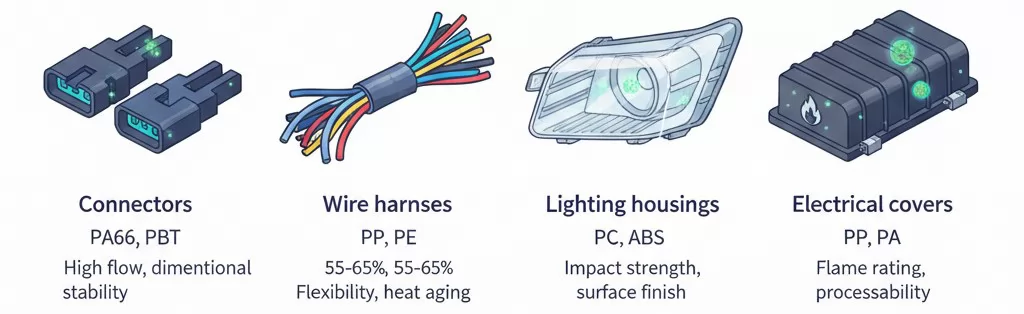
Typical automotive applications for MDH compounds:
| Component | Polymer | MDH Loading | Key Requirements |
|---|---|---|---|
| Connectors | PA66, PBT | 50-60% | High flow, dimensional stability |
| Wire harnesses | PP, PE | 55-65% | Flexibility, heat aging |
| Lighting housings | PC, ABS | 45-55% | Impact strength, surface finish |
| Electrical covers | PP, PA | 50-60% | Flame rating, processability |
Construction and Building Materials
Construction applications including pipes, profiles, sheets, and composite panels utilize MDH flame retardant compounds to meet building code requirements while providing durability and processability demanded by construction industry specifications.
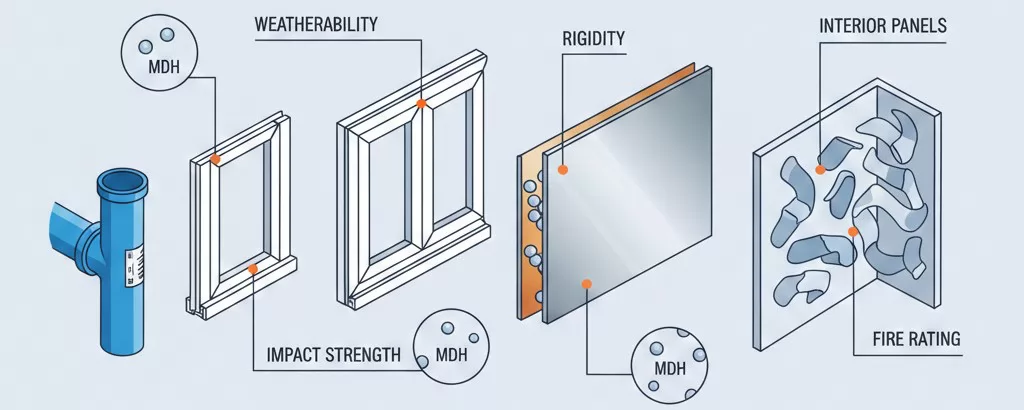
Key construction applications and requirements:
| Application | Processing Method | MDH Loading | Critical Properties |
|---|---|---|---|
| Pipe systems | Extrusion | 55-65% | Pressure rating, chemical resistance |
| Window profiles | Extrusion | 50-60% | Impact strength, weatherability |
| Aluminum composite panels | Extrusion | 60-70% | Surface finish, rigidity |
| Interior panels | Calendering/Extrusion | 55-65% | Fire rating, formability |
Conclusion and Technical Partnership
Magnesium hydroxide has established itself as a cornerstone of halogen-free flame retardant formulation, offering environmental advantages and thermal stability that make it suitable for demanding applications across wire and cable, automotive, and construction markets. However, the high loading levels required for effective flame retardancy create significant rheological challenges that manufacturers must understand and address to achieve successful processing outcomes.
Summary of key strategies for successful MDH compound processing:
- Surface treatment: The single most impactful intervention, reducing viscosity by 30-50%
- Particle optimization: Bimodal/trimodal distributions improve packing and flow
- Equipment configuration: Modified screws, reinforced dies, adequate drives
- Process parameters: Higher temperatures, pressures, and modified profiles
- Quality monitoring: Torque, pressure, and output consistency tracking
Successful implementation of MDH flame retardant compounds requires collaboration between material suppliers, compounders, and end-product manufacturers to optimize formulations for specific applications and processing systems. At KMT Industrial, we specialize in providing technical expertise and high-quality magnesium hydroxide products that enable our customers to overcome rheological challenges and achieve their performance objectives. Our team of technical specialists can assist with formulation development, processing optimization, and quality verification to ensure successful implementation across your product line. Contact our technical team to discuss your specific requirements and discover how our MDH products and expertise can enhance your flame retardant compounding operations.
Your Name*
Your Email*
-
2026-Jan-22AM3V: The Next-Generation Flame Retardant Replacing ATO in PVC CompoundsDiscover AM3V, KMT Industrial’s innovative flame retardant replacing ATO in PVC compounds. Achieve high LOI (≥30), low smoke density, and 1/15th the cost.
-
2026-Jan-19How Magnesium Hydroxide Affects Polymer Rheology and ProcessingDiscover how magnesium hydroxide (MDH) affects polymer rheology and processing. Learn expert solutions for optimizing extrusion, injection molding, and compounding of HFFR flame retardant compounds. Technical guide for polymer engineers and manufacturers.
-
2026-Jan-13How to Choose the Best Magnesium Hydroxide Flame Retardant SupplierSelecting the right MDH supplier is critical for HFFR performance. This guide covers technical grades, surface coatings, and global compliance. Learn more with KMT Industrial.


-

 +86-931-7653361
+86-931-7653361 Room 1212, 1213, Jinhe Building, No. 1264 Beibinhe West Road, Anning District, Lanzhou City, Gansu Province, China.
Room 1212, 1213, Jinhe Building, No. 1264 Beibinhe West Road, Anning District, Lanzhou City, Gansu Province, China. -
Quick Links
-
Products